Opioids: Concealing Commercialization
Several motivational forces are at play creating the controversial and contradictory nature of opioid pain medication. mypainweb feels it’s important to share this particular topic. I call it “Concealing Commercialization” because while this economic practice greatly influences our nation’s opioid healthcare crisis, it remains largely hidden from view.
The timing for the rollout of several new powerful super-opioids seemed unusual. The need for new, more effective, and safer opioids is crucial. During the past we’ve had a few new opioids brought to market from time-to-time, but nothing like the frequency we’ve seen recently. The off-the-chart strength of these new opioids appeared as a “red flag” to me. After reviewing the somewhat weak clinical data used to obtain FDA approval for these new opioids and viewing the increasing national opioid health concerns something did not make sense.
A quick analysis of the phenomenal growth of the pharmaceutical companies bringing these new powerful super-opioids to market was a blatant sign that our priorities and actions for reversing our tragic opioid trends are in the wrong direction. Subsys is the new powerful super-opioid I’m focusing on due to it’s most recent release date. This rationale is applicable for the many other new powerful super-opioids as well.
In the face of certain pushback from many that will find disagreement with this particular section, I’m confident many more will agree and support the complete facts.
The next few brief background sections are necessary to guide you to see with clarity that which is preferred by some to remain concealed:
Opioid: the Brief
Opioid therapy as a treatment option for long term Chronic Pain is at the center of a highly-emotional, bitter fight.
You can click on the link below to learn particulars of the argument:
During my 20 year Chronic Pain odyssey opioid medication has played a significant role in managing my pain levels. While I can’t write the empirical formula for Hydrocodone I do have 20 years of first-hand practical opioid knowledge and understanding. My direct experience with opioids includes a wide range of many different opioids along with several opioid delivery methods including oral pill, patch, oral transmucosal, buccal, and intrathecal pump.
Interested in reviewing the extensive list of my prescribed Opioids? Click on the link below:
mypainweb Prescription Medications
mypainweb contains an extensive array of opioid resources and information. For those living with long term chronic pain, opioids remain one of our most complicated and frustrating obstacles as we struggle to achieve some pain relief and quality of life. Opioid therapy is considered a last resort therapy for long term chronic pain.
Click on mypainweb non-intervention options to review alternative opioid options.
Recently there has been several new, powerful super-opioids approved, introduced and brought to market. At first glance they appear to be logical in response for even stronger opioids as it’s claimed the existing opioids fail to provide pain relief.
All types of pain are not equal
Understanding a few pain distinctions will help you understand the nature of our opioid healthcare crisis.
Pain as a result of cancer often requires the use of the most powerful available opioids as the goal is to insure “no pain” as the patient is nearing end of life. In the realm of palliative care / hospice for the terminal cancer patient we strive to preserve the patient’s dignity through delivering the most humane care with powerful opioids. This cancer pain group is rightfully excluded from the usual concerns associated with powerful opioid therapy.
Patients suffering from acute pain is pain that generally lasts for short duration (days to a few weeks not to exceed 6 months) may also have a need for stronger opioids. Acute pain would be result of an injury and, or surgery. Stronger opioids used for a finite, short duration can be used effectively and relatively safely.
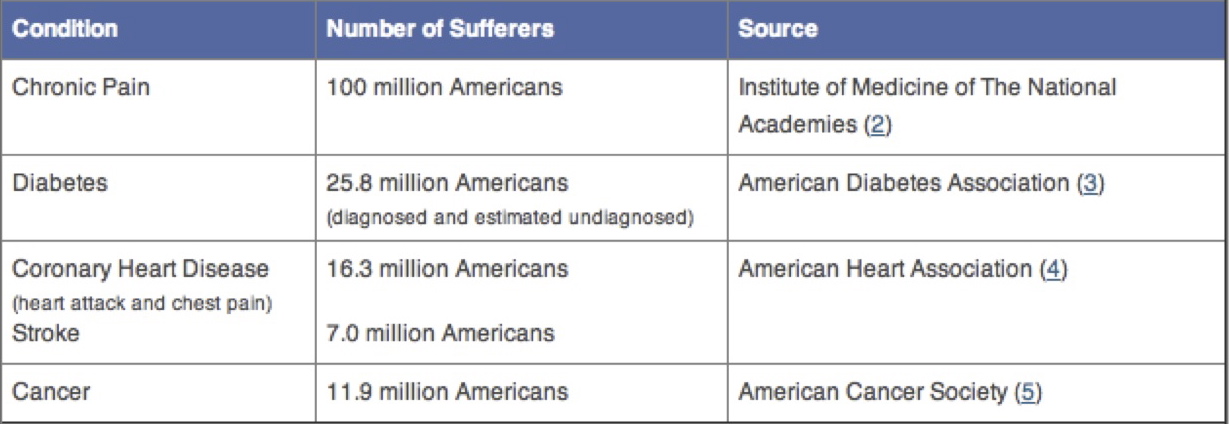
Acute pain patients and patients suffering from pain as a result of cancer represent a significantly smaller percentage as compared to those suffering with long term non-cancer chronic pain.
Click on the link below to read more:
It’s estimated that a total of 46 million people report acute pain which may last for a few hours but generally not lasting for more than 6 months. Pain continuing for longer than 6 months is considered long tern Chronic Pain.
Click on the link below to learn more:
At a minimum long term chronic pain outpaces acute and cancer pain about 2:1.
Opioid Commercialization
It’s all about size
The successful commercialization (financial gain/profit) of opioids is based upon an ever-increasing market. Chronic Pain is a prime opioid market which now affects 100 million Americans.
Exponential growth is expected into the future as the Opioid Commercialization success realized in the U.S. (along with a few other developed nations) goes global.
It’s estimated by 2025, there will be 1.2 billion people over the age of 60 suffering with Chronic Pain needing opioid pain medication. This huge market does NOT include those suffering with Chronic Pain under the age of 60. The potential growth for the global opioid medication market is unprecedented and lucrative to say the least.
New is better….isn’t it?
The recent introduction of yet another new powerful super-opioid, Subsys, demonstrates one of the quintessential problems which directly contributes to our national opioid healthcare crisis.
Wait…..How can our new powerful super-opioids be part of the opioid problem?
Efforts to reverse the devastating trends of opioid related health concerns are being hampered. Despite new, more powerful super-opioids, like Subsys, that are being created, marketed and prescribed the opioid health concerns continue to grow evidenced by the soaring numbers of addiction, depression and overdose deaths.
It’s “Opioid du jour”
The “opioid du jour” among pharmaceutical companies‘ uses the powerful opioid Fentanyl. Fentanyl is approximately 100 times more potent than morphine and many times more potent than heroin. Fentanyl is a potent, synthetic opioid that works very fast.
Fentanyl has been produced into many different opioid pain medications by several pharmaceutical companies. Fentanyl has been further evolved and differentiated by pharmaceutical companies by creating new delivery methods to get Fentanyl into the body. Here’s what I mean:
1. Patch….the traditional way to get fentanyl is via a patch that is applied to the skin that contains the fentanyl. The fentanyl is absorbed through the skin over several hours. Duragesic is a popular fentanyl patch.

2. Oral Transmucosal….this is basically a fentanyl lollipop that quickly dissolves in the mouth. Actiq is a popular brand.
3. Buccal....this is a fentanyl tablet that’s place on the gum line that quickly dissolves in the mouth. Fentora is a popular brand.

4. Nasal Spray….Fentanyl delivered in a nose spray form. Lazanda is a known brand.

5. Mouth Spray….Fentanyl delivered in a mouth spray form. Subsys is the new super-opioid.
The opioid complication quickly grows
New powerful-super opioids are not limited to fentanyl alone. Creating more powerful opioids through synthesizing other opioids like hydrocodone is a common practice,
Subsys, unfortunately, keeps company with other new recently approved powerful super-opioids such as Zohydro and Targiniq. Despite these new stronger opioid options more people are suffering with worsening Chronic Pain with increasing rates of opioid’s devastating side affects including addiction, depression and overdose deaths.
The glaring obvious conclusion is that opioids are not providing effective and safe pain relief. On the contrary, opioids are destroying more and more lives while establishing itself as a rock-solid lucrative business industry posting healthy quarterly earnings and dividends.
But we need more powerful opioids!
Subsys, like many others, starts with the claim from a pharmaceutical manufacturer that stronger opioids are needed…..Really? Says who? For What purpose?

We continue to push the opioid strength factor by “100 x’s even 1000 x’s more potent than it’s morphine equivalent”, yet the claim is made we need even stronger opioids. The pharmaceutical companies state the common goal that patients need more pain relief faster.
When stronger is not a good.
Keep in mind all opioids work by changing everything about the person so the person “feels” less, or no pain. Logically, stronger opioids just deepens that change within the person. The result is that the patient’s relief may be quicker from these new more powerful opioids. However; this is where we may see the “beginning of the end” for many. That “change within” which comes faster and deeper from these new powerful super-opioids is actually addiction and depression which can ultimately lead to overdose death, or a lifelong struggle with opioid medication’s disastrous side effects. In fact, many Chronic Pain sufferers report continued or even worsening Chronic Pain despite using more powerful super-opioids.
FDA’s seal of approval means it’s safe……right?
Thinking the FDA insures our safety by requiring rigid, extensive clinical trial testing? SInce the FDA approves the opioid medication, my doctor writes me the opioid prescription, and my neighborhood pharmacy fills the opioid prescription, then I should be confident in the opioid’s inherent “safety”….Right?
….Wrong. In the case for Subsys, FDA approval was based results from a clinical trial involving results from only 130 subjects. Positive results for adequate pain relief from 98 (75%) of the tested 130 subjects with tolerable side effects is all that was needed for FDA approval.
Click on the link below to learn more:
Small scale testing for these new super-opioids seem to be the norm. Your “uneasy” feelings are about to turn into “nausea”. It’s true that The FDA approves these opioids for a specific use. The F.D.A. approved Subsys only for cancer patients who are already using round-the-clock painkillers, and warned that it should be prescribed only by oncologists and pain specialists. But just 1 percent of prescriptions are written by oncologists, according to data provided by Symphony Health, which analyzes drug trends. About half of the prescriptions were written by pain specialists, and a wide range of doctors prescribed the rest, including general practice physicians, neurologists and even dentists and podiatrists.
You mean prescription medication, including opioids, can be prescribed for non-approved reasons?

Under F.D.A. rules, manufacturers may market prescription drugs only for approved uses. Since the FDA does not regulate how doctors practice medicine, doctors may prescribe drugs as they see fit. Over the last decade, pharmaceutical companies have paid billions of dollars to settle claims that they encouraged doctors to use drugs for non-approved treatments, or so-called off-label uses, to increase sales and profits.
A common “off-label” use of the newer opioids is prescribing them for non-cancer chronic pain patients though they were FDA approved for cancer pain patients. Through the eyes of many, “pain is pain”. That all patients suffering from pain should have access to these new super-opioids. In fact, some believe it would be “cruel and inhumane” to deny access of these newer opioids to all those in pain.
In the case of medications like Subsys, prescribers must undergo training about the drug’s risks, pass a test and register in a national database. Patients must sign an agreement with their doctor before being prescribed the drug. These action steps are intended to add some reassurance that Subsys will not be prescribed inappropriately, nor be misused/abused. Sounds good, but the assurances are empty as similar steps have been taken many times in the past with other opioids. If these steps were an effective deterrent, we would see a positive impact on the disastrous opioid trends.
Revealing Opioid Commercialization
Other factors which fuel our opioid national healthcare crisis in addition to “off-label” uses include questionable sales and marketing practices (promoting off-label uses) by some pharmaceutical corporations along with added incentives to pay out higher sales commissions to those that sell these newer opioids aggressively.

These opioid practices work as evidenced by the remarkable financial success of these pharmaceutical corporations. Insys Therapeutics, makers of the new super-opioid Subsys, has experienced sudden, soaring sales and share price of nearly 270%. Subsys sales continued to grow in the first quarter of this year, to $40.7 million, from $9.7 million a year ago.
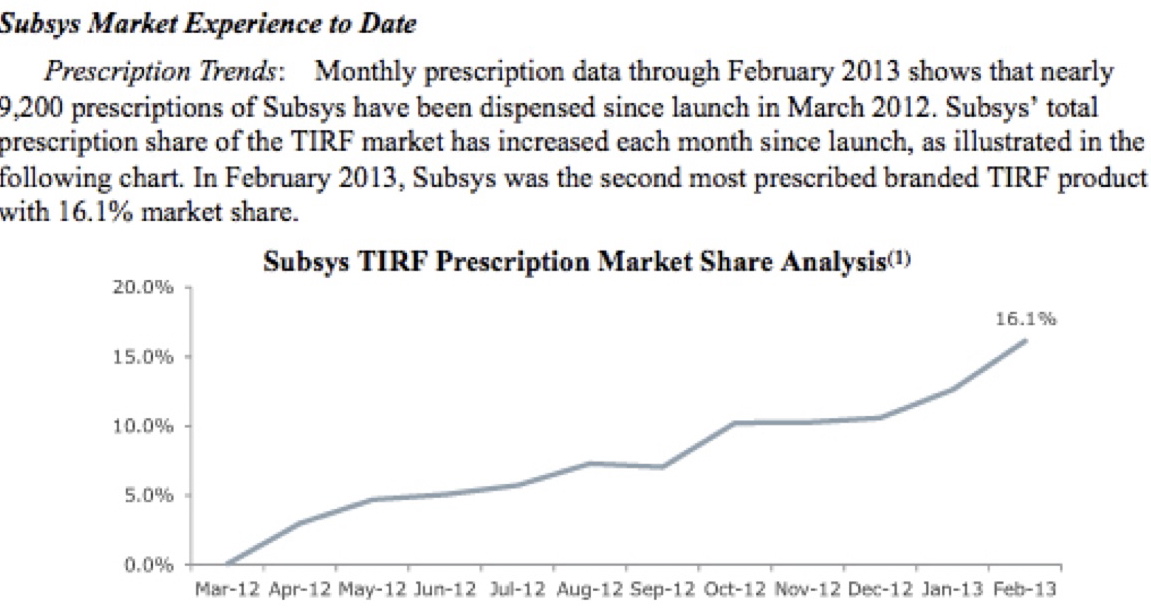
The sales growth grey-line (above chart) is hauntingly similar to the Overdose Deaths from Opioids red-line (below chart).
What do you think?
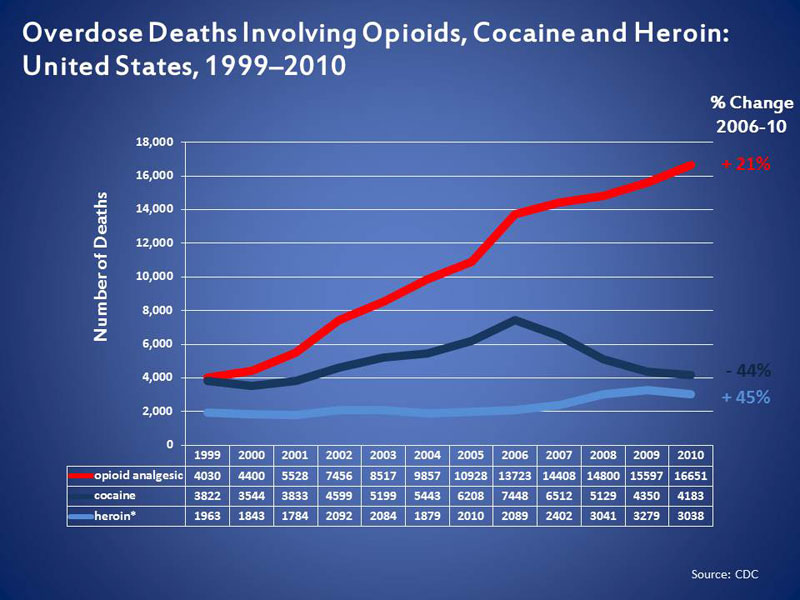
In fact, Insys Therapeutics plans for Subsys are only just getting underway: Earlier this year, Insys announced that it intended to seek approval to market the product (Subsys) for a broader range of uses, including for children and burn patients, and to treat acute pain in emergency rooms.
Click on the link below to read Subsys’ Prospectus:
Click on the link below to learn more about Subsys:
Doubts Raised About Off-Label Use of Subsys
One can quickly see why and how complicated our opioid healthcare problem has become.
Here are a few take aways to help understand and simply this opioid problem:
1. The realistic goal for pain is NOT take away all the pain, but reduce the pain level. Our expectation and desire to eliminate all our pain pushes the “opioid needle into the red”. The “opioid red zone” is the area where using powerful new-super opioids and, or higher dosages of stronger opioids increases the risk of addiction, depression and overdose death. Milder, much less potent opioids will work for longer periods of time and prove to be much safer.
2. Commercialization of the opioid industry is a key contributing factor explained as follows:
Below is my formula that correlates the impact the sales of powerful Opioids have on the tragic side affects from Opioids on Americans:
Off-label use + questionable marketing practices + aggressive selling = Addiction + Depression + Overdose Deaths from Opioids
Increasing values on the left side of the equation increases the values on the right side. Conversely, decreasing values on the left side of the equation decreases values on the right side.
Latest FDA New Super-Powerful Opioid is Approved November 2018
Dsuvia….another New FDA Approved super-powerful Opioid is on the market.
While the lengthy FDA Statement clearly states why society needs yet another super-powerful Opioid 1,000 X’s more potent than Morphine, it continues to put citizens at risk.
The FDA and AcelRX (manufacturer) must be held accountable & liable for even a single dose of Dsuvia that is found anywhere illegally. That includes cases where Dsuvia is “copied” (reproduced) illegallyand sold.
Click on the following link to read about Dsuvia
Opioid Reform is needed to finally change how this equation is being used.
A simple, yet effective start would be for Big Pharma to stop referring to patients as “annuity” income. Insensitive? In my opinion, absolutely.
Click on the following link to read how this label is misused:
Glaxo chief defies calls by activists for break-up
How disappointing that we must remind Big Pharma we are people. Would you buy a product from a company that refers to you as an “annuity”? Insulting and cold isn’t it??
Replacing the word “annuity” with “patient” would go a long way, and wouldn’t cost Big Pharma a dime….
Click on mypainweb Opioids for more Opioid information and details.
Click on mypainweb Opioid Solution for a solution to our national opioid healthcare crisis.
Here’s a glimpse that very well may be a possible future for Big Pharma’s lucrative opioid market…..
Purdue Pharma, the maker of OxyContin, filed for Chapter 11 bankruptcy protection Sunday night, just days after striking a settlement with more than 2,000 local governments over its alleged role in creating and sustaining the deadly opioid crisis.
The filing in New York follows the Sackler family agreeing to relinquish ownership of the lucrative company. The family also agreed to provide $3 billion in cash over several years and future revenue from the sale of OxyContin to assist communities hardest hit by the opioid epidemic.
Purdue Pharma filed for Chapter 11 bankruptcy protection
