CDC announces and introduces new Opioid Prescribing Guidelines:

Click on the following CDC link to read specifics about the Opioid Prescribing Guidelines:
CDC Opioid Prescribing Guidelines

Moments after posting the Opioid Abuse Bill (below)…..the following article is published explaining Painkiller politics:

Painkiller Politics makes one point crystal clear: agreement exists among politicians, Big Pharma, and special interest groups to not solve our Chronic Pain-Opioid Addiction epidemic.
New proposed Opioid Legislation:




Change at the Top Requested at the FDA

Anti-addiction groups call for the FDA Commissioner to step down.
Finally, a key step in the right direction.
Click on the following link to read more:
Call for the FDA Commissioner to step down
New Rules for Hydrocodone
After 10 years of formal debate and 15 years since the idea was first proposed, the strong opioid hydrocodone combination painkillers are finally being reclassified from the more-permissive Schedule III to the more-restrictive Schedule II category.
The change, effective October 6, 2014, won’t solve all the overuse/misuse problems associated with this potentially addictive opioid narcotic. This important step will help reduce the tragic accidental overdose death rate from opioid painkillers which now claims over 16,000 Americans annually.
Hydrocodone was already rightfully categorized as Schedule II. Oddly, in 1971 hydrocodone (at lower strength) when combined with another pain-killing drug, such as acetaminophen, was allowed to be placed in the less-restrictive Schedule III. In retrospect, it appears that this particular controlled substance legislation proved to be absolutely wrong as it contributed to our current national opioid overdose epidemic.
Click on the following link to read more:

Another powerful opioid approved by the FDA without the usual and customary Advisory Committee Vote

Click on the following link to learn about the new “abuse-deterrent” controversial powerful opioid called Targiniq:
Opioid pain medication may soon be regulated
“Why should I care about the Opioid epidemic problem?” “The Opioid epidemic problem affects a select-few-celebrity-types and drug addicts.” “I’m neither, so it’s not my problem” you say…..well, think again.
If you or a family member visit your dentist regularly, or seek medical treatment from your primary care physician for a painful knee injury, sprained ankle, etc., then YOU MUST CARE.
What’s the Opioid epidemic about?
*Deaths:
Each day, 46 people die from an overdose of prescription painkillers in the US with another 54 people dying everyday from illegal drug overdosing. Overdosing is now the leading cause of accidental death in the United States, accounting for more deaths than traffic fatalities or gun homicides and suicides. Fatal overdoses from opiate medications such as oxycodone, hydrocodone, and methadone have quadrupled since 1999, accounting for an estimated 16,651 deaths in 2010.
Click on the following links to read more:
100 Americans die of drug overdoses each day
*Increasing availability of opioids:
In 2011, about 131 million prescriptions for hydrocodone-containing pain medications were written for about 47 million patients, according to government estimates. That amounts to about 5,000,000,000 (billion) pills; just one opioid pain medication in just one year!
Click on the following link to read more:
FDA urging tighter rein on painkillers
Between 1991 and 2010, prescriptions for opioid analgesics increased from about 75.5 million to 209.5 million.
Click on the following link to read more:
*Where you live in the US influences opioid availability:
U.S. health care providers wrote 259 million prescriptions for opioid painkillers in 2012, enough to give a bottle of the pills to every adult in the country. But your chances of ending up with those pills – and the risks that come with them – depend a lot on where you live, says a new report from the federal Centers for Disease Control and Prevention.
Click on the following link to read more:
Prescription Painkiller vary widely among states
*Cost:
It is estimated that substance abuse costs the United States in excess of $600 billion annually in health, crime and lost productivity costs — and this is nothing compared to the toll it takes on the families, friends, schools and communities affected.
Click on the following link to read more:
Under the proposal passed by the House on Wednesday, March 12, 2014, schools within the State of Ohio would be required to update their health curricula with information about the addictive properties of prescription opioids and their links to heroin.
Click the link below to learn more:
Ohio’s prescription painkiller abuse health curricula
White House report on Opioid Overdoses:
More Americans are using and dying from prescription painkillers than from heroin. According to the Centers for Disease Control and Prevention (CDC), we’ve seen roughly a 20 percent increase in overdose deaths involving prescription painkillers since 2006. In 2010, there were over 16,000 drug poisoning deaths involving prescription painkillers. There were about 3,000 drug poisoning deaths involving heroin that same year.
Why are opioids potentially unsafe??
Here are a few of the well-known reasons:
Here is the NOT well-known reason:
Prior to obtaining FDA approval, research is done on all medications to produce evidence of the long-term effectiveness and safety of medications. Particular scrutiny is given to medications used for managing long-term chronic conditions.
Person-time is the research measurement used to estimate the actual time in years, months, or days that all persons contributed to the study-observation of a particular medication. Analysis of the person-time results is used to determine the safety & effectiveness of the trialled medication.
Check out these examples:
Hypertensive medications…………..1,800,000 person-years were studied
Cholesterol medications……………..750,000 person-years were studied
NSAIDS (aspirin & ibuprofen)……100,000 person-years were studied
Opioids ………………………………………1,500 person-years were studied
Opioids by comparison, have only a meager person-years observation. The studies of chronic opioid therapy are too short to evaluate long-term effectiveness, and too small to adequately evaluate safety. Source: Opioids for Chronic Pain
Opioids, though FDA approved, are one of the most potentially dangerous medications prescribed today has actually been studied the least. What do you think?
The Zohydro fight intensifies and get’s ugly as the argument takes on a politicizing slant. Once again, creating distraction from the logical decision adds complication & delay. Click on the following link to read the full story: Heroin in a Capsule?
Feel Safer??
The highly controversial & potentially dangerous super-opioid ZOHYDRO (now available for sale) will be offered in bottles with combination locks!!
Click on this link to read more: Feel Safer??

Massachusetts takes “UNPRECEDENTED ACTION”…bans sale of FDA-approved Zohydro super-opioid painkiller!!
Click the following link to read more: Ban Zohydro

Zohydro Manufactured by Same Company That Makes Addiction Medicine
OUTRAGEOUS!!
Zohydro is made by the same company, Zogenix, that manufactures Vivitrol, a drug used to TREAT patients ADDICTED to opioids as reported by The New York Times.
So, we’re ready to unleash this dangerously powerful painkiller while we’re trending more deaths each year from prescription drug overdose than automobile traffic accidents!
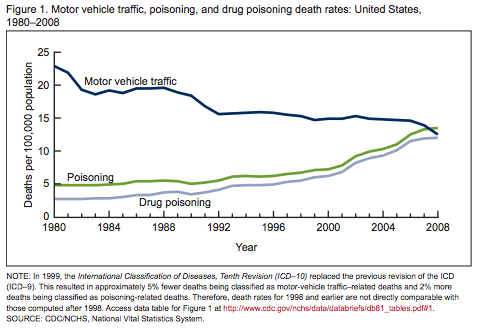
Click the following to read more: Overdose rates
What is Zohydro?
It’s a super-opioid painkiller that has been FDA approved, shipped and ready for sale. Zohydro is 10 x the power of other painkillers like Vicodin. A child could die from ingesting just one capsule & an adult new to opioids could die from just 2 capsules. Check out Zohydro’s Warning Label below:


Why was Zohydro approved?
The Food and Drug Administration (FDA) claims that daily, around-the-clock, long-term treatment cannot be treated with our arsenal of existing available painkillers.

Click the following link to read more: Zohydro
Safety Claims:
Zogenix, Zohydro’s manufacturer, is publicizing its efforts to make sure the drug is used safely. The company’s approach involves the use of “safety monitoring boards”. Exactly who will be monitored (patients, doctors, or pharmacies) and how will they monitor (reviewing confidential doctor-patient medical files, dispensed prescriptions, sales data, direct contact) has not been disclosed. Experts say there is little evidence that the so-called “safe board” will ensure Zohydro is used appropriately. Critics point out the fact that 5 out of 7 experts on the “safe board” have previously received consulting payments from the company. All “safe board” panel members will be compensated for quarterly meetings to review Zohydro data. In essence, the claim has been made stating this external “safe board” panel of independent people is not independent, but are working directly for Zogenix. Furthermore, critics raise concern about the “safe board’s” level of authority or power whatsoever.
Click the following link to read more: Zohydro Safe Board
Zogenix’s multiple roles in the world of painkillers is troubling to say the least.
One thing that is certain……the revenues & profits to be generated from the new super-opioid painkiller Zohydro along with their opioid addictiondrug, Vivitrol, will insure a HEALTHY balance sheet and ROI for Zogenix.
The FDA approves ZOHYDRO……the new SUPER Opioid Painkiller.
* 10 x the power of other painkillers like Vicodin
* A child could die from ingesting just one capsule &
an adult new to opioids could die from just
2 capsules
* Nearly 60 percent of all drug overdoses are from FDA-
approved prescription medications. Zohydro will likely
trigger a spike in deaths caused by opioids. Keep in mind
in 1999 about 4,000 people died from opioid poisoning
with 17,000 deaths in 2010!!
* Over 40 Industry experts along with Congressional
Representatives & State Attorney Generals urged the
FDA to say NO to Zohydro.
* The FDA chose NOT to listen to its own advisory panel,
which voted 11-2 AGAINST approving Zohydro
* Why did the FDA approve Zohydro? Zogenix, the drug
company maker, argued its safer than the existing opioid
alternatives because it doesn’t contain acetaminophen.
Zohydro is expected to be available next month.
Government Oversight
Government Oversight effort is gaining momentum as Congress introduces legislation to provide increased Federal oversight of prescription opioid treatment and assistance to States in reducing opioid abuse, diversion, and deaths.
On February 13, 2013 the 113th Congress, First Session, introduced H.R. 672: Prescription Drug Abuse Prevention and Treatment Act of 2013. Please click on the following link to review the complete H.R. 672: Prescription Drug Abuse Prevention and Treatment Act of 2013: H.R 672.
On January 25, 2013 an advisory panel to the FDA recommends additional restrictions on opioids as reviewed in this link: FDA Panel Recommendations.
Further complication resides in the fact that each State has several types of laws dealing with the practice of opioids. The following link contains all such laws for each State: Centers for Disease Control and Prevention.
In the State of California, a bill known as AB Assembly 889 recently passed out of Assembly Appropriations Committee. This legislation was amended in the State of California on May 2, 2013. This important legislation will give people suffering from chronic pain quicker access to needed pain medication as prescribed by their healthcare provider without the usual obstacles and delays from insurance carriers. The following link will provide particulars of this legislation: California’s AB 889.
Interestingly, pain research accounts for only about 1% of research grants awarded by the National Institutes of Health (NIH) while the 2012 annual cost of chronic pain is as high as $635 Billion a year which exceeds yearly costs for cancer, heart disease and diabetes combined. The following link reviews the details: Federal Spending to Treat Chronic Pain.
The American Academy of Pain Medicine reports that the most common type of chronic pain is low back pain. This group represents 27% of the 100 million Americans suffering from chronic pain. Second place is tied with chronic pain from severe headaches, or migraines 15% and neck pain 15%. It’s no surprise that chronic low back pain and chronic neck pain may have a direct physiological correlation. These top two chronic pain categories (low back pain and neck pain) represent 42% of all chronic pain sufferers. The following link provides more specifics: AAPM.
Despite the significant size of chronic low back pain and neck pain sufferers, the National Institutes of Health (NIH) does not spend a single penny for research in this particular category. Keep in mind that the NIH spends $30.9 billion annually for medical research with just 1% allocated for all chronic pain. The top 2 causes of chronic pain (low back pain and neck pain) don’t get one cent.
The following link lists all the NIH medical research spending by category: NIH Categorical Spending.
How about a warranty on implanted medical devices? Read about this important topic at Implanted Medical Device Warranties.
Read the following link to gain insight on the FDA’s likely future direction concerning Opioids: Long-Term Opioid Use Questioned for Chronic Pain
mypainweb will continue to monitor Government Oversight as it relates to chronic pain and post updates as they unfold.